Medicine supply
A marketing authorisation works to ensure that when a medicine reaches a patient it works in the same high quality way every time.
Medicines are highly regulated and the Medicines and Healthcare Products Regulatory Agency (MHRA) issues a ‘marketing authorisation’ for every medicine.
The marketing authorisation governs not only what the medicine is for and how it should be used, but also the details of the formulation, storage requirements and shelf life, so that the medicine that reaches patients works and is of the same high quality every time.
To learn more about the work of the MHRA, visit the Licensing of medicines section of the MHRA website.
The MHRA also issues ‘manufacturing authorisations’ which entitle a company to manufacture medicines in the UK. Companies need to demonstrate that they comply with good manufacturing practice and pass regular inspections of the manufacturing site. To find out more about this, visit the MHRA website.
The ABPI works through its Pharmaceutical Quality Expert Network to monitor regulatory activity on these topics and participate in the development of legislation at both national and European levels. The Network also facilitates the exchange between members of good practice in these important areas.
Environmental health and safety
Good environmental health and safety performance is a major consideration in all aspects of pharmaceutical industry activity.
Through the Environmental Health and Safety Network, we monitor regulatory activity on these topics and participate actively in the development of legislation at both national and European levels, as well as facilitating the sharing of good practice amongst member companies.
Medicine supply shortage
Every day, people in the UK rely on thousands of licensed medicines supplied by the pharmaceutical industry. Producing these medicines involves strict safety and quality standards within complex global supply chains.
Unfortunately, sometimes the supply of medicines fails to meet demand.
This can happen for various reasons, including manufacturing issues, disrupted access to raw materials or bottlenecks in distribution or transport networks.
Sometimes there is regulatory intervention or a batch failure where products fail to meet high quality control standards.
Companies maintain reserves of products to protect patients from shortages. The size of these is based on assessments of patient safety, future demand and foreseeable disruptions that might impact the supply chain.
When shortages occur, companies are required to notify the government, which works with stakeholders, swiftly address and resolve these shortages.
We work with the government, NHS and others to proactively strengthen supply chain resilience and effectively manage disruptions.
The Government has established the National Supply Disruption Response, which provides a single point of contact to maintain the supply of medical products to the UK.
There are also serious shortage protocols that allow local pharmacists to dispense an alternative to a prescribed medicine if there's a serious shortage.
These measures ensure that the medicines you rely on remain safe, effective and available.
Every day, people in the UK rely on thousands of licensed medicines supplied by the pharmaceutical industry. Producing these medicines involves strict safety and quality standards within complex global supply chains. Unfortunately, sometimes the supply of medicines fails to meet demand. This can happen for various reasons, including manufacturing issues, disrupted access to raw materials or bottlenecks in distribution or transport networks. Sometimes there is regulatory intervention or a batch failure where products fail to meet high-quality control standards.
Companies maintain reserves of products to protect patients from shortages. The size of these is based on assessments of patient safety, future demand, and foreseeable disruptions that might impact the supply chain. When shortages occur, companies are required to notify the government, which works with stakeholders to swiftly address and resolve these shortages.
We work with the government, NHS, and others to proactively strengthen supply chain resilience and effectively manage disruptions. The government has established the national supply disruption response which provides a single point of contact to maintain the supply of medical products to the UK. There are also serious shortage protocols that allow local pharmacists to dispense an alternative to a prescribed medicine if there's a serious shortage. These measures ensure that the medicines you rely on remain safe, effective, and available.
Managing medicine shortages
Every day millions of patients rely on medicines supplied by the pharmaceutical industry.
Complexity of medicine production and supply
The UK relies on thousands of licensed medicines and more than half a million different medical technology products.
Producing these medicines involves strict safety and quality standards within complex global supply chains. Unfortunately, occasionally the supply of medicines fails to meet the demand.
Supply shortages can happen for a variety of reasons, including manufacturing issues, disrupted access to raw materials, bottlenecks in distribution or transport networks, regulatory intervention or a ‘batch failure’ which is where products fail to meet the high quality control standards expected in medicine manufacture.
Companies maintain reserves of products to protect patients from shortages. The size of these reserves is based on a company’s assessments of future demand, alongside any foreseeable disruptions that might impact the wider supply chain. This planning is easier for medicines which come from a single company, for example, an on-patent branded medicine, as they have full visibility of the market. It can be much more complicated to predict necessary reserves for medicines with multiple suppliers, as is the case with generic medicines. Knock-on impacts from one supplier can impact the ability of other companies to cover any shortfall. Occasionally, a sudden change in prescribing patterns can cause unexpected spikes in demand, leading to a shortfall in supply. Another factor might be a complication further up the production supply chain, which impacts multiple suppliers all at once.
Finally, when a new medicine is launched, it can take time to scale up production to meet global demand. Occasionally expanding production can meet with unexpected challenges which take time to address.
Protecting patient from supply shocks
To protect patients it is important for the government, manufacturers and suppliers to proactively identify threats to supply, strengthen supply chains resilience and effectively manage disruptions when it occurs.
This requires an approach that covers the whole of the supply chain – from the gathering of raw materials, through to manufacturing, storage and distribution.
Achieving this requires sustained and effective efforts with international partners, government, the NHS and independent contractors.
Understanding medicine shortages
When shortages happen, companies are required to notify the Department of Health and Social Care (DHSC) who will work with a range of stakeholders to swiftly address and resolve these shortages using tried and tested procedures.
The DHSC has published guidance which outlines the responsibilities that manufacturers and suppliers have to inform the department of any medicine discontinuations or anticipated supply shortages, to help manage supply issues and help prevent any potential impacts on patients.
Companies take their responsibilities very seriously and are accountable to UK regulators should supplies of their products become unavailable.
HRT medicines shortages
The government has worked with the industry to improve supply of HRT products.
The ABPI established a dedicated Project Team of our members to focus on supply chain issues that relate to HRT products. By volume, the HRT market is mainly generic, but we are in contact with government and other trade associations on issues relating to supply.
We are also working closely with our members, the NHS England Menopause Programme and the DHSC Medicines Supply Team to support supplier engagement on HRT and wider Women’s Health Strategy.
You can see our last statement on HRT here.
Addressing shortages
Although shortages in individual products are inevitable in complex supply chains, there are established processes set by DHSC and the NHS for reporting and responding to shortages of medicines and medical products.
-
DHSC is responsible for the continuity of supply of medicines and manufacturers have a legal requirement to inform DHSC of any supply problems.
-
DHSC has introduced the DaSH portal for Marketing Authorisation Holders to submit notifications to the DHSC Medicine Supply Team relating to potential medicine shortages, discontinuations or updates on current medicine supply issues.
-
DHSC has established the National Supply Disruption Response (NSDR), which acts as a single point of contact to maintain supply of medical products to the UK.
-
DHSC have also instituted an Express Freight Service contract to provide emergency logistics for any medical or medical product from anywhere in the world.
-
The Serious Shortage Protocols (SSPs) allows for local pharmacists to dispense an alternative to a prescribed medicine if there's a serious shortage.
-
DHSC reviews its list of medicines that cannot be exported from the UK or hoarded by wholesalers because they are needed for UK patients.

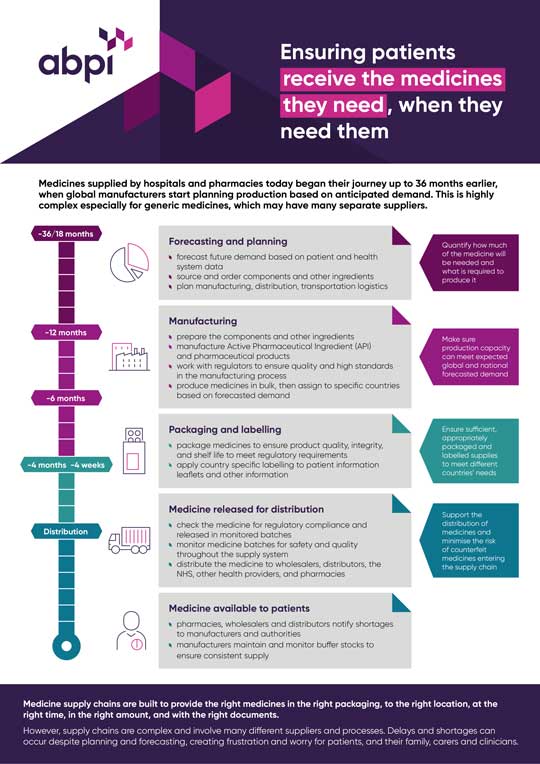
Managing medicine shortages infographic
We have produced a simple resource that shows the journey a medicine takes to reach patients and how medicine shortages can occur.
Counterfeiting
The manufacture and selling of counterfeit medicines is an issue of increasing concern throughout the world. There are potentially fatal consequences for those who inadvertently take such products.
The ABPI and its member companies work very closely with the Medicines and Healthcare products Regulatory Agency (MHRA) to develop effective measures to prevent the entry of counterfeit medicines into the UK. We have been very active in the development of a new European Directive which it is hoped will be adopted in the near future and which will provide stricter controls on manufacture, supply and trading of medicinal products which will provide greater protection against this dangerous trade.

Related publications
Careers - job case studies related to medicine supply
-

Supply Chain Apprentice
I found the AZ's AT apprenticeship posted on the government website and immediately applied as I saw, it was a great opportunity even though it was almost three hours away from my hometown. Since then, I've loved it. I'm happy so I believe and I'm really happy that they're more than the advertised as honestly it's such a great scheme.
-

Clinical trials supplies
My first experience of the pharmaceutical industry was as a 12-week summer placement at Pfizer in their early formulation department.
-

VP Global Clinical Supply Chain
I lead the team that is responsible for the manufacture and the management of clinical supplies that feed into all our ongoing clinical trials
Last modified: 30 September 2025
Last reviewed: 30 September 2025