About us
The Northern Ireland Confederation for Health and Social Care (NICON) is the voice of the organisations working across Northern Ireland’s integrated Health and Social Care (HSC) system. Part of the UK-wide NHS Confederation, NICON is the only membership body for all HSC organisations.
NICON’s membership comprises all six HSC Trusts (including the Northern Ireland Ambulance Service); the ‘regional’ organisations (the Public Health Agency and the Business Services Organisation); as well as the smaller, ‘specialist’ HSC bodies.
Additionally, NICON’s associate membership scheme is open to public, commercial, and not-for-profit organisations working in the health and social care space.
To find out more, visit www.nhsconfed.org/nicon

The Association of the British Pharmaceutical Industry (ABPI) exists to make the UK the best place in the world to research, develop and access medicines and vaccines to improve patient care.
We represent companies of all sizes that invest in making and discovering medicines and vaccines to enhance and save the lives of millions of people around the world. In England, Scotland, Wales and Northern Ireland, we work in partnership with governments and the NHS so that patients can get new treatments faster and the NHS can plan how much it spends on medicines.
Every day, our members partner with healthcare professionals, academics and patient organisations to find new solutions to unmet health needs.
To find out more, visit www.abpi.org.uk
Foreword
The Association of the British Pharmaceutical Industry (ABPI) and the Northern Ireland Confederation for Health and Social Care (NICON) have a shared ambition: to harness the power of partnership to improve care, accelerate innovation, and deliver real, lasting value for people, systems, and society. We are therefore delighted to offer this guidance, which is built on strong and trusted collaboration between our two organisations.
The Department of Health NI, published a Reset Plan in July 2025, setting out how health and care services must drive forward rapid change and one key element of this is creating new models of working—models that break down organisational silos, align shared goals, and unlock the strengths each partner brings.
This guidance responds to that need. It offers a practical and values-led framework for building partnerships that are strategic, sustainable, and focused on what matters most—better outcomes for patients and the population. It invites health and industry partners to think boldly, act collaboratively, and deliver with purpose.
The evidence for partnership working is clear. When done well, it delivers what has become known as the “triple win” — benefits for patients, for the health system, and for industry. Research by Carnall Farrar supports this.
Farrar has shown how collaborations can be associated with better clinical outcomes. These outcomes are not theoretical — they’re already being delivered across the UK, in areas like pathway redesign, digital innovation, and population health management. The ABPI’s expanding library of case studies showcases what effective partnerships can achieve, and how success can be replicated. The King’s Fund has further underlined the importance of trust, transparency, and shared purpose as essential foundations.
In Northern Ireland, ABPI in partnership with the Medicines Optimisation Innovation Centre (MOIC) developed the Health and Social Care Industry Partnership (HSCIP), a bespoke framework endorsed by the Department of Health. It offers a regionally supported, transparent approach to engagement, aligned with integrated care priorities and designed for sustainable, long-term impact. The routes through which partnerships are undertaken via the HSCIP are clearly referenced in this guidance, providing practical direction for organisations seeking to collaborate with purpose and accountability.
This guidance challenges us to raise our sights, to commit to collaboration not as an exception, but as a norm. By embracing the principles set out here, we have a real opportunity to shape a future in which partnerships are not only effective, but transformative.
Now is the time to act – together.
Northern Ireland Confederation for Health and Care
ABPI
About this guidance
This guide provides a practical, step-by-step resource to help the Health and Social Care (HSC) system and industry develop, deliver and evaluate partnerships that will drive improvements to the health and wellbeing of patients in Northern Ireland (NI).
It contains templates, recommended frameworks, and handy checklists and prompts to support you. As well as national guidance it includes the NI bespoke HSC Industry Partnership framework, developed in 2020 in partnership with the Medicines Optimisation Innovation Centre (MOIC) and endorsed by the system which aims to support regional Industry partnerships within NI. MOIC is a regional centre in NI dedicated to delivering medicines optimisation to the people of NI.
Access downloadable, editable documents wherever you see the icon.
Who this guidance is for
This document is aimed at the HSC, industry members, and those leading on the partnership and transformation agenda within their organisations or systems.
We want you to use it to:
bring stakeholders and partners together
to assess priorities
design and implement partnership projects aligned to strategic objectives
and informed by the guidance resources
strengthen assurance and nurture the culture
of effective partnership working
scale existing partnerships
across care settings
At a glance: How to develop effective HSC-industry partnerships
Who this guidance is for
Healthcare organisations in NI, industry leaders and those leading on the partnership and transformation agenda within their organisation or system.
Use it to:
bring stakeholders and partners together to assess priorities
design and implement partnership projects aligned to strategic objectives and informed by the guidance resources
strengthen assurance and nurture the culture of effective partnership working
scale existing partnerships across care settings.
Project identification and scoping
Key activities
Identify unmet need: Healthcare organisations and industry partners identify unmet needs to support improved clinical outcomes and explore how collaborative working and joint working projects can assist. This process includes community engagement and patient feedback to identify opportunities for improvement.
Assess scope: Undertake assessment of the project scope and aims to help identify whether a project is a collaborative or joint working project.
Agree objectives and timescale: Outline the project’s purpose, objectives, resource impact and timelines – to be approved by parties to enable progression to Stage 2, the project setup stage.
Project setup and governance structures
Key activities
Form project team: Project team or steering committee is formed.
Build trust: Work undertaken to engender trust and ethos of effective partnership working.
Develop project initiation document (PID): Develop and approve a project initiation document (PID) that details the aims and objectives of the project, expected outcomes, project completion date, exit strategy, project organisational structure, resources as well as data and patient protection.
Governance frameworks: Establish project governance arrangements
Engage with stakeholders: Undertake stakeholder engagement relevant to the project setup.
Certify PID: PID developed and agreed with the healthcare organisation, project team, governance committee and industry partner respectively. It must then be certified by the industry partner.
Publish executive summary: An executive summary of the project rationale, period, objectives, roles and responsibilities of the parties, and financial arrangements must be published on the industry partners website before arrangements are implemented. It should be certified by the industry partner.
Project implementation and outcomes reporting
Key activities
Deliver project: Project delivery and evaluation.
Monitor progress: Project monitoring and regular reporting against outcomes outlined in the PID and within the written agreement. If significant changes to the project occur during implementation, an amendment framework should be completed and agreed by the project team and the governance committee.
Publish project outcomes: All parties should publish outcomes of the project within six months of completion.
Disclosure UK: Transfers of value related to projects must also be disclosed via
Deliver project: Project delivery and evaluation.
Monitor progress: Project monitoring and regular reporting against outcomes outlined in the PID and within the written agreement. If significant changes to the project occur during implementation, an amendment framework should be completed and agreed by the project team and the governance committee.
Publish project outcomes: All parties should publish outcomes of the project within six months of completion.
Disclosure UK: Transfers of value related to projects must also be disclosed via the Disclosure UK database.
The 2024 ABPI Code of Practice exists to regulate the promotion of prescription medicines to UK health professionals, industry interactions with health professionals, and the provision of information about prescription-only medicines to the public.
The benefits of working together
Partnerships offer a proven ‘triple win’ — improving outcomes for patients, strengthening the health system, and supporting responsible industry innovation. In NI, where health inequalities, service variation, and workforce pressures remain major challenges, these partnerships offer a strategic opportunity to accelerate transformation:
Equitable and evidence-based prescribing
Carnall Farrar’s analysis of partnerships showed that NHS organisations engaged in industry collaboration were 2.5 times more likely to follow NICE recommended, clinically and cost-effective prescribing – particularly in cardiovascular care.
Capacity and Capability for Transformation
The King’s Fund report highlighted how partnerships enable frontline clinicians to lead change — supported by industry-funded project management, analytics, and digital tools. This boosts staff capacity while embedding sustainable service redesign.
In NI, where stretched teams often lack time for innovation, this external support can be transformative.
Improving outcomes and reducing inequalities
Collaborations with the life sciences industry have consistently been shown to enhance access to best-practice care, improve population health outcomes, and reduce unwarranted variation.
In NI, where health outcomes remain closely tied to deprivation, partnerships offer a mechanism to support early intervention and better management of chronic conditions in the communities that need it most. By aligning clinical practice with national guidance and enhancing access to diagnostics, medicines, and preventative services, these partnerships help close the health gap between more and less advantaged areas – a key priority for NI’s integrated care systems.
The ABPI Code of Practice
The 2024 ABPI Code of Practice exists to regulate the promotion of prescription medicines to UK health professionals, industry interactions with health professionals, and the provision of information about prescription-only medicines to the public.
It is administered by the Prescription Medicines Code of Practice Authority (PMCPA) and is the cornerstone of the UK system of industry self-regulation. All NHS-industry partnerships are bound by the ABPI Code of Practice.
“NHS-industry partnerships are bound by the code, which serves as a guardrail by which industry is regulated to ensure that throughout all collaborations, patient safety is maintained, in a professional, ethical and transparent manner to ensure the appropriate provision of high-quality care.” Dr Amit Aggarwal, Executive Director, Medical Affairs and Strategic Partnerships, ABPI
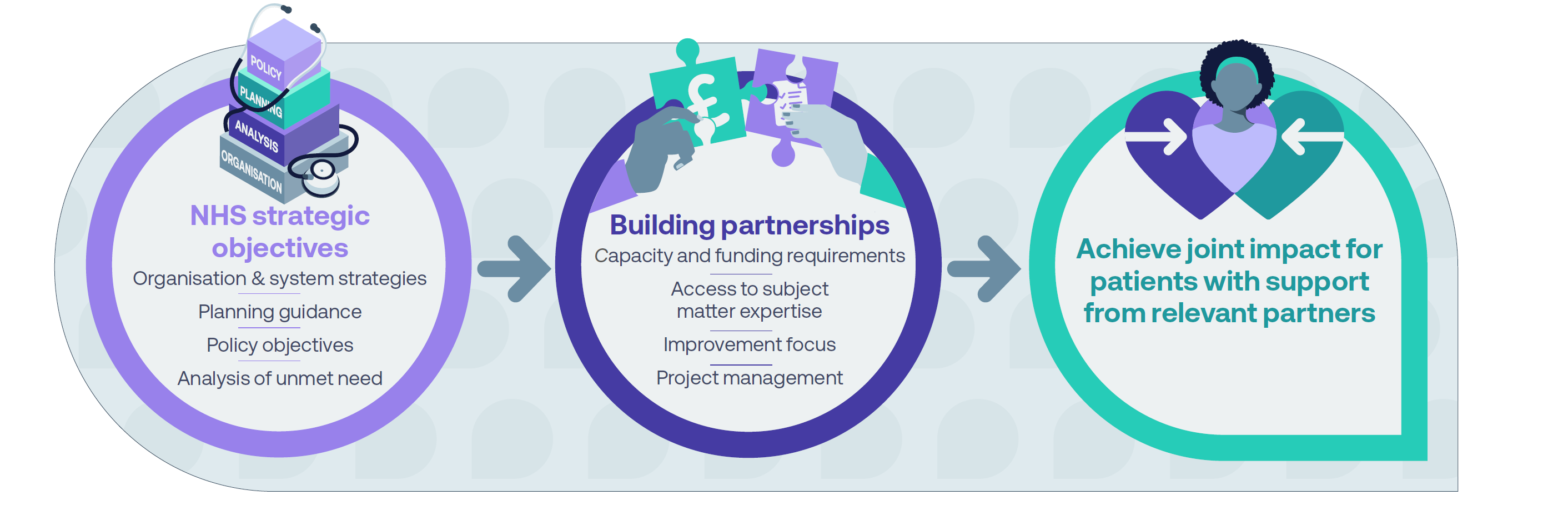
What are healthcare system/industry partnerships?
Healthcare system/industry partnerships allow healthcare systems and the pharmaceutical industry to collaborate for patients’ benefit.
Joint working guidance was established nationally in 2008, when the Department of Health and Social Care (DHSC) acknowledged the value of external expertise in helping NHS organisations overcome challenges. This includes providing additional skills and resources to achieve patient benefits beyond that which NHS organisations could deliver alone. These partnerships bring industry skills and expertise to improve appropriate patient access to innovative treatments, support project management and enhance the efficient delivery of healthcare services.
Partnerships can be formed between a single healthcare system entity and a single pharmaceutical company, or multiples of either.
While patient organisations cannot directly be included in collaborative working arrangements, they may be contracted to deliver a service to support an element of such collaborative working.
There are two types of healthcare system/industry partnerships: collaborative working and joint working projects. These partnerships involve cooperation between industry and across primary, secondary and system-level healthcare settings.
Collaborative working with organisations must enhance patient care or be for the benefit of patients, or alternatively benefit the NHS/HSC and, as a minimum, maintain patient care.
There must be a shared commitment to successful delivery from everyone involved and each organisation must make a significant contribution. In the case of healthcare organisations, this contribution does not have to be financial. It can involve the sharing of support in the form of skills and experience to deliver projects successfully.
Joint working is a limited form of collaborative working which must be patient-centred and always benefit patients directly.
Collaborative and joint-working approaches: a comparison
Benefits for pharmaceutical companies in embarking upon collaborative working can be myriad, from gaining experience in partnering with a healthcare organisation, to an increase in patient identification and prescribing in accordance with national and local guidelines.
Most importantly however, and in accordance with Clause 20 of the ABPI Code of Practice, such benefits must not constitute an inducement to health professional or other relevant decision makers to prescribe, supply, recommend, buy or sell a medicine. A key safeguard here is the requirement in the ABPI Code of Practice to have a summary of the collaborative working agreement publicly available before arrangements are implemented.
It is also worth noting that in embarking upon collaborative working with healthcare organisations, companies will have no direct contact with patients, or with identifiable patient level data.
| Collaborative-working projects | Joint-working projects |
|---|---|
| Are for the benefit of patients and/or the healthcare organisation, including the NHS. | Must always be for the benefit of patients directly and must include the NHS as a party. |
| Enhance patient care or be for the benefit of patients, or alternatively benefit the NHS and, as a minimum, maintain patient care. | |
| May not constitute a grant/donation (see Clause 23 of ABPI Code of Practice for further information on Donations and Grants). | |
| May provide benefits to the company or companies involved. | |
Outcomes must be defined in such a way that they can be measured or tracked, so that at any time during the collaboration all parties are aware of:
|
|
| Must be carried out in an open and transparent way, with a certified summary of the project agreement publicly available before it begins. | |
| Must respect clinical independence. | |
| Must be prospective – not relating to a project that has already begun. | |
| Must have the value to the healthcare organisation publicly disclosed annually on the Disclosure UK database and, if relevant, the contracted service value to the patient organisation published on the industry partner’s website. | |
| Must not constitute an inducement to health professionals or other relevant decision-makers to prescribe, supply, recommend, buy or sell a medicine. | |
| Must ensure that the rights and legitimate interests of all parties are continuously observed throughout, including considerations related to data security, the protection of confidentiality and privacy, and anti-bribery compliance. | |
| Must not promote a prescription-only medicine to any member of the public. | |
Stage 1 Project identification and scoping
The figure below outlines the stages of a partnership, from planning to delivery and monitoring:
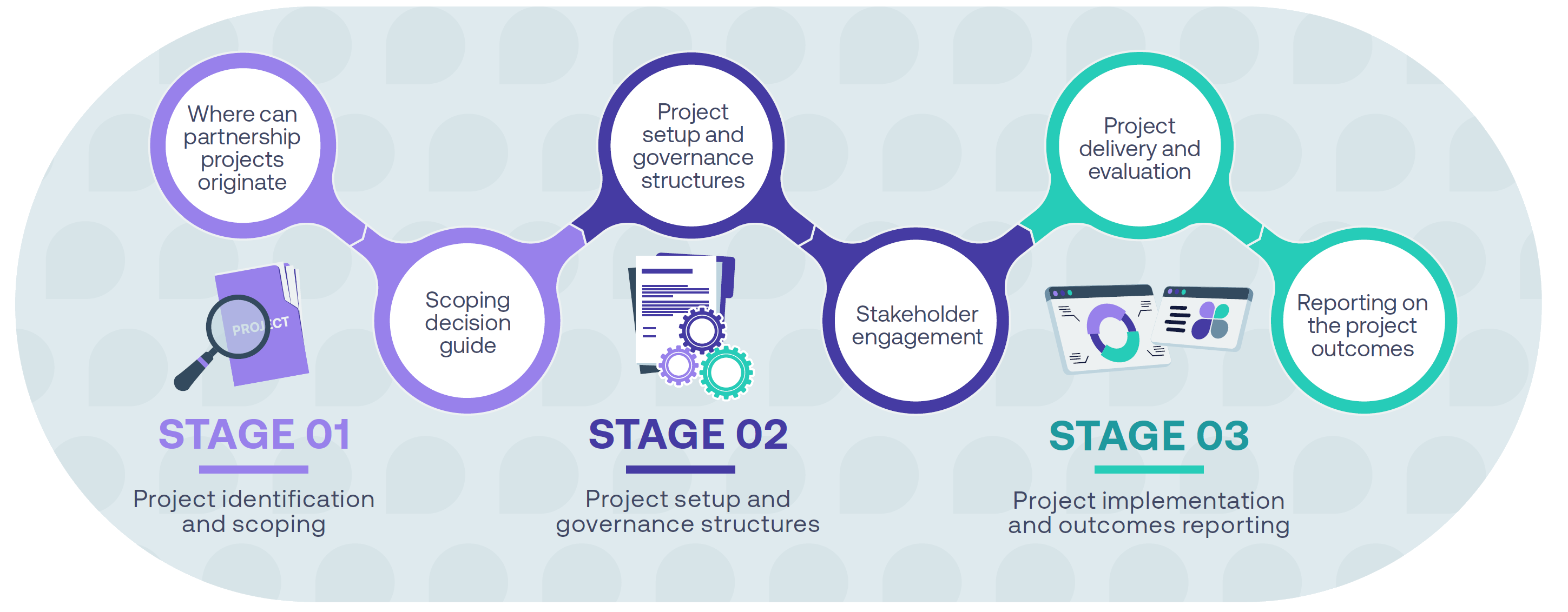
and scoping
“Effective partnerships between the HSC and industry can help deliver better patient outcomes, tackle health inequalities and accelerate the pace and scale of improvement. By working together we can harness innovation, share expertise, and ensure that every patient benefits from progress.”
Chief Pharmaceutical Officer, Department of Health NI
Where can partnership projects originate?
In NI, partnerships can originate from across HSC, as well as Industry. The table below sets out some suggestions. HSC staff can use MOIC services to support with initial project ideas.
Partnerships can also be applicable for NI that have originated in other parts of the UK for example, in integrated care boards (ICBs) or health innovation networks (HINs) in England or health boards in Scotland and Wales.
| Primary care | Secondary care | Trust level | SPPG | Industry | Region-wide |
|---|---|---|---|---|---|
| Primary care settings, GP federations. |
Secondary care settings, clinical networks, consultant-led. Business managers/ service leads. |
Partnerships may take place across one of the five health trusts within NI. This project can cover all entities within that trust as appropriate or may focus on a particular setting. | The Strategic Planning and Performance Group (SPPG), formerly the HSC board, oversees multi-agency partnerships and networks. | Industry will often research and directly scope out potential projects, upscale existing projects or widen projects over multiple geographical areas. | Projects that have the potential to be region wide for NI, can be routed through the HSC Industry Partnership with the support of MOIC (See page 18 and 19 for further details). |
Key resource: Scoping decision guide
At the start of a partnership, healthcare and industry organisations need to identify unmet needs to support clinical outcomes.
Industry partners often align their capabilities with HSC goals or issues identified through health data analysis
This process includes community engagement and patient feedback to identify opportunities for improvement.
Colleagues should use these questions to explore and develop the scope of a project, to ensure the aims are clear and the entire lifecycle
of the project has been considered.
| Questions to consider during the scoping of a project | Questions to consider if the project has a therapy/medicine focus |
|---|---|
| What is the unmet need the project is seeking to address? | Which clinical pathway(s) require service redesign to improve patient care and/or system improvement? |
| What is the scale of the problem? | |
| What is the evidence to back this up? | Which companies have expertise in this area? |
| What is the priority improvement area, and can this realistically be addressed within the resources and duration of the project? | |
| What are the patient or system benefits of addressing this need? | Are there other organisations that would be relevant to engage in the project? For example, at an ICS level, can HINs play a convening and facilitation role for the partnership? |
| What would be the impact on patient care or the system if this need is not addressed? | |
| Are other internal stakeholders supportive of addressing the need and the feasibility of doing so? | |
| Which NHS plan and/or local improvement plan goal is the challenge aligned to? | |
| What interventions are required and in what timescale? | How are partners considering the impact of health inequalities and equality of healthcare access? |
| What is the likely impact on the clinical and non-clinical workforce? For example, will this project involve complex pathway redesign? |
|
| Are prospective partners clear on existing operational pressures, and their potential impact on the project? |
|
| What related challenges need to be addressed in other parts of the system for the project to succeed? |
|
| What does success look like? How will it be measured? When and by whom? | |
| Is the project intended to demonstrate a sustainable solution? If so, what outcomes will be necessary to ensure a successful business case? |
|
| Is there capacity to release the necessary internal team members to participate in the project? |
Key resource: Checklist for determining if a project is collaborative working, joint working or neither
To help identify if a project is a collaborative or joint-working project, an
assessment of the project scope and aims should be undertaken, which can be supported by completing the checklist below.
If the answer to any red questions on the checklist is ‘No’, the project is not a collaborative or joint–working arrangement, and will need to be modified before proceeding. If changes cannot be made, prospective partners should consider an alternative approach, such as a research collaboration or a donation/grant, as described in Clause 23 of the ABPI Code of Practice.
If the answer to any amber questions is ‘No’, this signals an issue or risk that should be addressed to encourage successful and timely project delivery.
| Joint working | ||
|---|---|---|
| 1A. Is the main benefit of the project focused on the patient? | YES This is a joint-working project – go to question 2 (page 17) |
NO Please go to question 1B |
| Collaborative working | ||
| 1B. Does the project aim to enhance patient care or be for the benefit of patients, or alternatively benefit the NHS and, as a minimum, maintain patient care? | YES This is a collaborative-working project – go to question 2 (page 17) |
NO Consider another form of support |
| Red questions | |||
|---|---|---|---|
| # | Question | YES | NO |
| 2 | Do all parties acknowledge that the arrangement may benefit the NHS and company partner(s) involved? | YES | NO |
| 3 | Are any subsequent benefits at an organisational level and not specific to any individual? | YES | NO |
| 4 | Is there a significant contribution of pooled resources from all parties, which include people, finance and equipment wholly or partly dedicated to the project? | YES | NO |
| 5 | Is there a shared commitment to joint development, implementation and successful delivery? | YES | NO |
| 6 | Will anonymised, aggregated, outcome data be measured and documented? | YES | NO |
| 7 | Are all partners committed to publishing a measured and documented executive summary of the Collaborative Working Agreement? | YES | NO |
| 8 | Are all proposed treatments involved in line with national guidance, where it exists? | YES | NO |
| 9 | Will all activities be conducted in an open and transparent manner, with appropriate governance arrangements in place to manage any conflicts of interest? | YES | NO |
| 10 | Has an exit strategy and any contingency arrangements been agreed? | YES | NO |
| Amber questions | |||
|---|---|---|---|
| # | Question | YES | NO |
| 11 | Will the project be managed by a team including representatives of industry, the NHS and appropriate third-party representation? | YES | NO |
| 12 | Do all parties and their respective organisations have the appropriate skills, capabilities and capacity to manage the project? | YES | NO |
| 13 | Have all partner organisations got clear procedures in place for reviewing and approving collaborative-working projects? | YES | NO |
| 14 | Are all parties committed to working together across the entire lifecycle of the partnership? | YES | NO |
| 15 | Are all partners clear on who within their organisation is responsible for ensuring that relevant joint-working / collaborative-working documents are certified or approved? | YES | NO |
| Green questions | |||
|---|---|---|---|
| # | Question (add text) | YES | NO |
| 16 | Add green question here… | YES | NO |
| 17 | Add green question here… | YES | NO |
| 18 | Add green question here… | YES | NO |
| 19 | Add green question here… | YES | NO |
| 20 | Add green question here… | YES | NO |
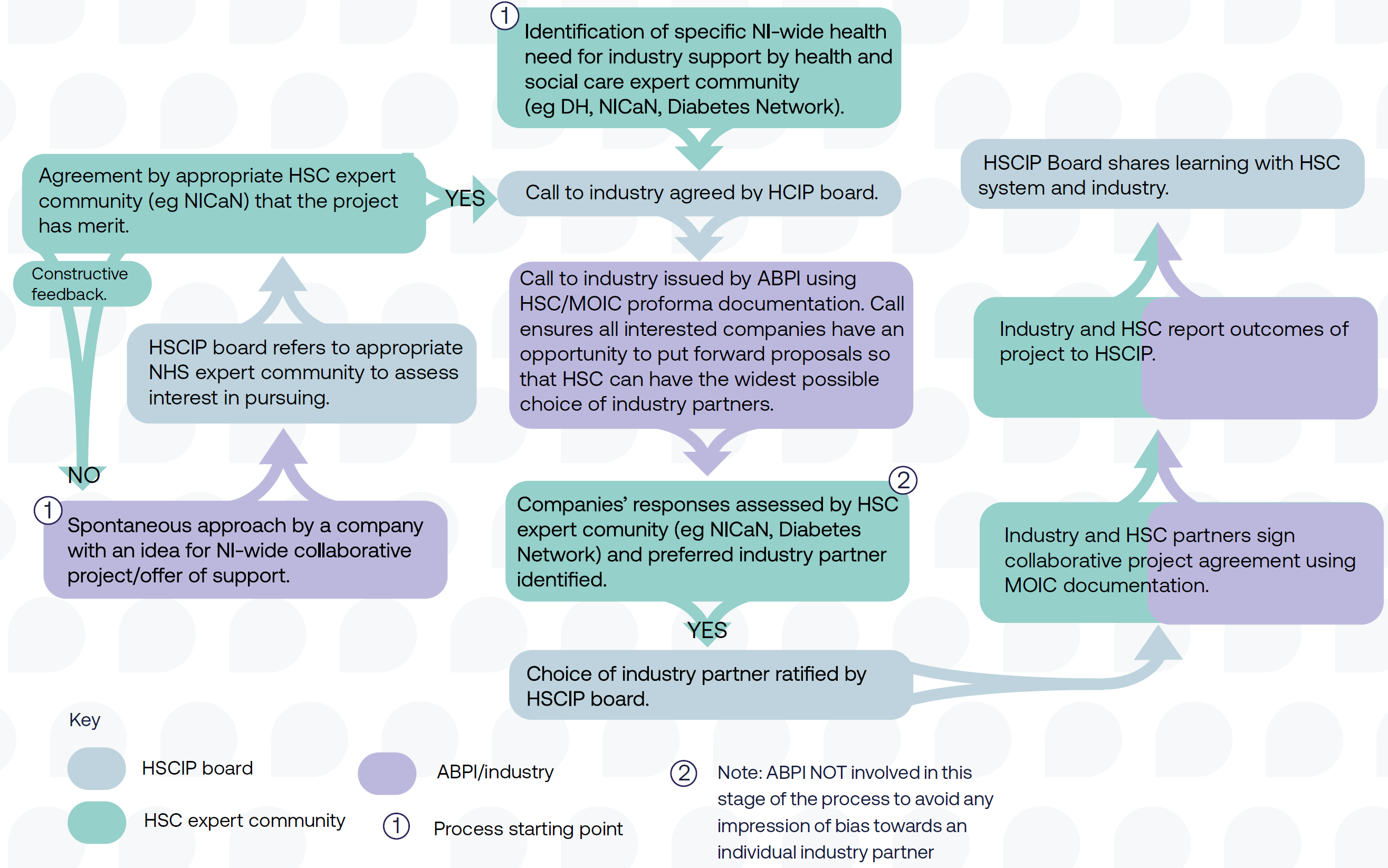
How the HSC Industry Partnership works
- The HSC Industry Partnership is a framework that was developed to support region-wide Industry Partnerships in NI, with the support of MOIC (see Appendix 5).
- The framework sets out a structured and governed process for project identification, partner selection, project management, delivery and evaluation, utilising MOIC’s established governance process and MOIC’s ability to act on behalf of HSC.
- The HSC Industry Partnership is overseen by a steering group, including leaders within HSC and the Department of Health.
- Potential projects are identified and scoped within the HSC system, or from Industry. • Projects are assessed on their suitability for the framework, this takes place between MOIC and the project originators.
- If suitable an ‘Invitation to Respond’ is issued to ABPI member companies. This document details the project and sets out the asks.
- Companies complete this document setting out their suitability and experience, returning these to MOIC.
- Potential partners are shortlisted and interviewed by MOIC and the relevant HSC leads
Health and Social Care Industry Partnership (HSCIP) board
Key resource: Project Concept Framework
The key recommended documentation for stage 1 is a Project Concept
Framework, outlining the project’s purpose, objectives, resource impact and timelines. It must be approved by all relevant parties to support progression to the project setup stage.
Invitation to respond: call to industry – Health and Social Care Industry Partnership
| Name of applicant company | |
| Submitted by (Name of accountable director) |
|
| Author(s) | |
| Submission date | |
| Introduction | |
| Aim of opportunity | |
| Scope of opportunity | |
| Timelines | |
| Budget | |
| Engagement approach | |
| Evaluation of applicants and selection criteria | |
| Project specific questions | |
| Governance | |
| ABPI Code compliance | |
| Staff and resources | |
| Document management | |
| Confidentiality and data protection | |
| Additional information | |
Stage 2 Project setup and governance structures
and governance
structures
Project setup and governance structures
At this stage, a project team or steering committee is formed, including all involved parties and active participants. This team will guide and manage the project, being responsible for its success and operating within the agreed limits. The members of this project team may differ depending on the care setting. The team should be ‘right sized’ to be both effective and inclusive.
All project team or steering committee members must declare any conflicts of interest. Those with a conflict should not vote on related matters. These declarations should be recorded in the meeting minutes. Either party can object to someone’s involvement due to a conflict of interest.
The project team should agree on a project methodology. PRINCE2 (Projects In Controlled Environments) is perhaps the best-known and most widely used, but methodology may vary depending on the complexity of the project. The team should also agree on a regular meeting schedule to make initial decisions, keep the project on track, and manage any issues.
Meetings should be action-oriented, with clear agendas, decision points, and minutes recorded.
When considering project resourcing, all parties must commit to detailing the resources they will contribute to the project, which could include finances, skills, or experience. These contributions should go beyond normal day-to-day roles, like funding additional staff or clinics. Assigning a monetary value to healthcare resources is challenging, but it should be clear that contributions from partners should be comparable and proportionate.
If the collaboration aims to address healthcare organisation constraints, precautions must be taken when using pharmaceutical funding to retain staff. Any staff paid through industry funding should operate under HSC control, be time limited and outlined in collaborative working documentation, with clear employment law compliance. Exit strategies for industry funded posts should be outlined in project documents.
Key resource: Project team/steering committee members (non-exhaustive)
Who should be in a project team/project steering committee? (non-exhaustive)
| Care setting (options depending on local context) | Key stakeholders |
|---|---|
| Primary care |
Industry
|
|
|
| Secondary care | |
|
|
| System-level care | |
|
Key resource: Steps to build trust
Trust and transparency is fundamental to any partnership, especially during the early scoping phase. All parties should ensure that they work towards engendering trust, which can be supported through the following steps:
Key resource: Project initiation document (PID)
Once the necessary criteria have been fulfilled, the project team should develop and approve a project initiation document (PID) to ensure a shared understanding of the project’s outcomes, its governance framework and to provide a clear exit strategy that details the overall responsibility of each party if the project needs to be terminated.
The PID is a key document that sets out requirements ahead of project implementation and the agreed copy should be kept on record for both parties’ reference.
Confidentiality of patient information must be maintained in all partnerships, as outlined in the PID. This includes respecting the confidentiality of project-related information and not sharing it beyond the project’s scope. The PID should be collaboratively created by relevant individuals from all partnering organisations.
It is important to note that PIDs will, at times, need to be updated following the initiation of the project. This can be addressed
via a project amendment form.
The PID must be certified by the industry partner and be approved by both the project team also the relevant governance committee(s) – see page 27 for further details.
Accelerating transformation: Project initiation document template
*Clause 20 of the ABPI Code states that collaborative working, including its implementation, must have and be able to demonstrate the pooling of skills, experience and/or resources from all of the parties involved for the joint development and implementation of patient and/or healthcare centred projects. There must be a shared commitment to successful delivery from all parties, and each party must make a significant contribution.
Establishing a robust governance framework
When entering into a partnership initiative, industry and NHS organisations need to ensure they are working within a robust governance framework to ensure the project aligns with their organisations’ goals and legal processes.
In NI, the MOIC team provide advice and support with governance requirements and can act on behalf of the HSC.
Projects should include the establishment of a governance committee to oversee the project. The governance committee will also review the principles of the project against the collaborative and joint-working checklist criteria and ensure that the project has been reviewed by each participating organisation’s management and experts.
Within pharmaceutical companies, governance expertise will be provided by legal, medical, compliance and healthcare engagement functions.
Within healthcare organisations, governance will usually be provided by existing governance committee or Internal Review Committee (IRC). For collaborative projects, one must find stakeholders with the authority to approve the project.
Key resource:
Potential governance committee members across care settings (non-exhaustive)
- Named executive senior responsible officer from each party to the partnership
- Relevant clinical lead
- Programme management and support
- Insight and intelligence teams
- Pharmaceutical legal director
- Pharmaceutical medical / compliance director
Stakeholder engagement
Building confidence in the project with stakeholders, both internal and external, is vital to avoid misunderstandings and ensure transparency.
This includes clarifying aligned interests and disclosing benefits to industry transparently. Clear communication between partners is essential to refine project objectives, manage expectations and confirm inputs from each organisation. At this stage, the project team should develop a stakeholder map, communications plan and data collection plan if not already done in the PID. Realistic timescales should be set, with the first three steps taking four to six months, including scoping, development and approval of governance arrangements and legal framework.
It is a helpful exercise to divide stakeholders between internal and external stakeholders who:
- should be involved in the project
- are not directly involved in the project, but whose views could influence the outcome and who should be kept informed throughout the entire project lifecycle
- will ultimately be impacted by the outcome
- whose opinions could facilitate or prevent success.
Key resource: Examples of stakeholder groups to engage across care settings (non-exhaustive)
| Primary care | GPs, pharmacists, patients and residents, and voluntary and community sector organisations |
| Secondary care | Clinical leads, chairs of trusts, trust directors |
| System-level care | SPPG directors/leads, Department of Health leads |
| Industry | Company decision makers, market access teams, project managers |
Patient engagement
HSC and industry partners should give due consideration to the impact on patients and, if appropriate, gain feedback from patients or patient groups.
Ways to incorporate patients’ experiences can include:
- Patient stories of their experiences
- Mapping key service pathways with patients and staff working together
- Ensuring community representation to reflect diversity and address inequalities
- Recording patient experience and capturing feedback for project outcomes
Key resource: Recommended Collaborative/Joint Working Agreement Framework
Once the project has been approved in principle by all relevant parties, the project team must work with its organisational legal experts to draft and sign a Collaborative/Joint Working Agreement. The agreement is a legal contract that will include key information about the project and plans, drawn directly from the PID. It must be entered into with legal, corporate entities and not with any individual in primary, secondary and system-level settings.
The agreement must ensure that any confidential, competitive or personal data are protected by strong contractual provisions. It should include the following:
- The name of the collaborative-working project, the parties to the agreement, the date and the term of the agreement
- Aims and objectives
- Considering community representation so that plans being developed represent diversity and the needs of different groups impacted and ensuring inequalities are considered
- The expected benefits for patients, the population or user groups, the NHS or other healthcare organisation, the pharmaceutical company and other organisation(s) as applicable
- Principal activities and accountabilities
- Composition of the steering group / project group
- Timelines and project milestones
- Description of pooled resources
- Financial arrangements
- Roles and responsibilities of the healthcare organisation, the pharmaceutical company and other organisations
- How the success of the project will be measured, when and by whom
- Relationship, if any, to the company’s or companies’ medicine(s)
- An executive summary of the project which will be published on the industry partner’s corporate website before the project begins
- Process for project amendment
- Dispute resolution clause
- Defined exit strategy (for all parties)
- Contingency arrangements to cover unforeseen circumstances (e.g. updated guidance or product summaries)
- Agreement on intellectual property rights following completion of the project
- Data management and data sharing plans
- Pharmacovigilance plans (if required)
- A plan to publish project outcomes within six months of completion to support wider learning
- A commitment to disclose transfers of value to healthcare organisations via Disclosure UK
Please note: Any collaborative or joint-working agreements must be entered into with legal, corporate entities and not with any individual member of staff across primary, secondary and system-level settings.
The executive summary
The last stage in the project setup is the publication of an executive summary, which will largely draw from the content in the collaboration working agreement.
As outlined in the ABPI Code of Practice, a summary of the colloborative working agreement (executive summary) must be published on the pharmaceutical company’s or companies’ website before arrangements are implemented. It is also advisable that relevant NHS partners do the same. The project should not commence until the executive summary has been published on the relevant industry partner(s)’ website. A recommended executive summary framework is shown in the key resource:
Key resource: Recommended Collaborative/Joint Working Project Executive Summary Framework
Accelerating transformation: collaborative / joint working executive summary template
Stage 3 Project implementation and outcomes reporting
and outcomes
reporting
“This partnership provides a great vehicle for
us to collaborate with our industry partners
in addressing challenges that face the health
service and so deliver better care for our citizens
improving the healthcare system and adoption
of the industry innovation.”
Medicines Optimisation Innovation Centre (MOIC)
Project delivery and evaluation
Once the collaborative agreement is signed and published on the industry partners’ website, the project officially starts.
To monitor project progress effectively, partners should refer to the outcomes outlined in the PID and executive summary.
Collaborative and joint-working projects are not set up as clinical trials or real-world evidence-generating trials, and as such, the project metrics need to be realistic and based upon the objectives of the project and intended purpose thereafter, ie business case or scalable solution. Examples of relevant metrics include clinical impact, delivery, service effects and economic impact. After project completion, outcomes will be measured and documented, with stakeholders and the project team evaluating learnings from the project.
Key resource: Project monitoring guidance
If significant changes to the project occur during implementation, the following form should be completed and agreed by the project team and the governance committee in order to keep a record of the project aims as part of good governance:
Key resource: Recommended Project Amendment Framework (for use only if necessary)
Reporting on the project outcomes
It is important to recognise that successful organisations will learn from their experiences of partnerships.
Learning is more beneficial when it is preserved beyond the end of the project in the outcome report. As well as evaluating the outcome of the project, it is useful to assess how successful or unsuccessful the operation of the project has been so that lessons can be learned and can be usefully applied in the design and running of other projects.
As stated in the ABPI Code of Practice, all parties should publish outcomes promptly, within six months. Local NHS organisations are encouraged to do the same. To promote and expand successful collaborations in healthcare, these outcome reports should be shared with ABPI for their NHS-Industry Partnership Case Studies Library to support more partnerships.
To ensure that partnerships are transparent, transfers of value related to collaborative projects must also be disclosed via the Disclosure UK database (see Appendix 2 for further details on Disclosure UK, including how any contracted service values to patient organisations are disclosed).
Key resource: Recommended Summary of Project Outcomes Framework
Summary of project outcomes: template
| Summary of project outcomes – template | |
|---|---|
| Project name | Include a short sentence that outlines the project title. |
| Project partners | Outline the partnering organisations that took part in the collaboration. |
| Duration | Detail the project initiation and completion dates to the nearest month, e.g. December 2025 – July 2026. |
| Project overview | Provide an overview of the project, including its objectives and how the project set out to achieve them. |
| Project outcomes | Outline the results from the project, including benefits to patients, the NHS, and industry. This section should address the following:
|
| Conclusions and learnings | Include the conclusions and learnings drawn from the project, with the aim of supporting learnings for other projects. |
| References | Include examples of any healthcare organisation or National Institute for Health and Care Excellence (NICE) policies that are relevant to the project. |
Further reading
Previous ABPI/NHS Confederation publications
- Collaborate to Innovate: Learning from NHS, Charity and Life Sciences Industry Experience to Build a Culture of Research and Innovation in the UK (April 2024)
- Partnering with Purpose: How Integrated Care Systems and Industry Can Work Better Together (November 2023)
- Transforming Lives, Improving Health Outcomes: Tackling the True Cost of Variation in Uptake of Innovative Medicines (January 2023)
- Accelerating Transformation: How to Develop Effective NHS-Industry Partnerships*
Current guidance
Appendices 1, 2, 3 and 4
Appendix 1: Criteria for HSC Industry Partnership
The Health and Social Care Industry Partnership (HSCIP) is a partnership agreement with the Medicines Optimisation Innovation Centre (MOIC) and the Association of the British Pharmaceutical Industry (ABPI). Through this partnership, parties work together to introduce transformative, medicines-related innovations across the Health and Social Care (HSC) system. The shared goal is to deliver ‘triple win’ benefits to patients, HSC and the economy through more rapid and consistent patient access to innovation, more effective use of HSC resources and increased cross-sector research collaboration.
- HSCIP projects are collaborative partnerships to improve the health outcomes for patients.
- HSCIP projects can originate from all the partner organisations.
- Industry partners should be prepared to provide solutions and offers of support and to be able to commit to these proposals.
- HSC partners should be able to commit to developing the scope and scale of the projects, along with allocating a dedicated project team to oversee work.
- Projects will not be supported without clear and agreed project documentation setting out measurable aims, ways of working, organisational responsibilities, accountability, timelines and clear project management.
- Projects must be in areas of mutual interest to improve the diagnosis, treatment and care of patients.
- All parties agree voluntarily to work together to achieve the shared vision of measurably improved outcomes for people affected by identified conditions.
All partners acknowledge and fully support the wide range of legal and governance requirements that already exist to guide interaction between industry and the HSC. These include:
- UK and EU competition
- UK and EU competition law
- ABPI competition law guidance
- ABPI Disclosure UK process
- GDPR
Appendix 2: The ABPI Code of Practice
The 2024 ABPI Code of Practice exists to regulate the promotion of prescription medicines to UK health professionals, industry interactions with health professionals, and the provision of information about prescription-only medicines to the public, including patients and patient organisations.
It is administered by the Prescription Medicines Code of Practice Authority (PMCPA) and is the cornerstone of the UK system of industry self-regulation.
All NHS-industry partnerships are bound by the code, which serves as a guardrail by which industry is regulated to ensure that throughout all collaborations, patient safety is maintained, in a professional, ethical and transparent manner to ensure the appropriate provision of high-quality care. At its heart, the code gives confidence to local NHS organisations that partnerships operate in a clear and robust framework.
Strong support is given to the code by the industry with all companies devoting considerable resources to ensure that their activities comply with it. Any complaint made against a company under the code is regarded as a serious matter both by that company and by the industry as a whole. Sanctions are applied against a company ruled in breach of the code.
Underpinning this are the ABPI Principles, which sit alongside the code. These set out the behaviours that embody the spirit of the code, and the ABPI expects that companies build these into their culture and approach.
The four key principles are as follows:
- Commitment to benefiting patients and ensuring patient safety by operating in a professional, ethical and transparent manner to ensure the appropriate and rational use of medicines and to support the provision of high-quality healthcare.
- Acting with integrity and committing to relationships that are responsible, professional, ethical and transparent.
- Commitment to ensuring that transparency is respected.
- Interacting with all stakeholders with respect.
The ABPI Code reflects and extends beyond relevant UK legislation and ensures that the ABPI meets its commitments to implement other codes, such as those from the International Federation of Pharmaceutical Manufacturers and Associations and the European Federation of Pharmaceutical Industries and Associations Codes.
The code is also supplemented by Disclosure UK, a Europe-wide initiative to increase transparency between pharmaceutical companies and the organisations they work with. Further information on Disclosure UK can be found in Appendix 3.
Appendix 3: Disclosure UK
The relationship between the pharmaceutical industry, healthcare professionals and healthcare organisations plays a vital role in the development of life-enhancing and life-saving medicines.
At the core of the relationship is sharing knowledge to improve patient outcomes. To ensure this relationship is open and transparent, the pharmaceutical industry has taken the lead on disclosing ‘transfers of value’ – payments and benefits-in-kind – made by industry to healthcare professionals and healthcare organisations through Disclosure UK, a publicly searchable database hosted by the ABPI.
Disclosure UK is part of a Europe-wide initiative to increase transparency between pharmaceutical companies and healthcare professionals and organisations.
Data shown on Disclosure UK covers:
- Participation in advisory boards
- Speaking at or chairing meetings
- Working with and advising doctors and scientists in pharmaceutical companies
- Speaking at conferences and symposia
- Attendance at national and international conferences
- Medical education and training funded by companies
- Provision of grants and donations to healthcare organisations
- Sponsorship of healthcare education events
Details of collaborative and joint working projects with healthcare organisations are also disclosed individually on the database. Certain research and development transfers of value to healthcare professionals, decision makers, and healthcare organisations are also disclosed in aggregate.
Separately, companies must disclose transfers of value made to patient organisations and fees for contracted services to members of the public, including patients and journalists.
The ABPI Code requires that this information is published on company websites with gateway links to Disclosure UK.
For more resources or to search the database, visit: www.disclosureuk.org.uk
These disclosure requirements are in addition to:
- An executive summary of each project published on the industry partner’s website before project start.
- Outcome publications within six months of project completion by all partners.
Appendix 4: Multi-partner considerations
Collaborative working projects between a single healthcare organisation and a single pharmaceutical company are common. Less common but still possible are cross-sector projects involving more than one healthcare organisation and/or more than one pharmaceutical company.
Cross-sector projects between one or more healthcare organisations and one or more pharmaceutical companies are often more complex. Each organisation has its own governance and approvals processes, which can lengthen timelines for project set-up and implementation.
In addition, ABPI members may be actual or potential competitors in certain therapy areas and are therefore subject to stringent competition-law safeguards that must be reflected in any agreement on working together.
A shared desire to improve patient outcomes through collaboration will not justify anticompetitive conduct (e.g., illegal exchange of sensitive information between competitors or other collusive practices).
There should be a lead contracting party (by role) in each sector when multiple healthcare organisations are involved, or where an individual contract with each organisation is required.
Where there is more than one contracting party on each side, steps should be taken to understand differing legislative competence, governance processes and timelines.
Given the higher legal risk and opportunity for error when multiple organisations are involved, it is best to agree rules of engagement upfront and record them in the agreement. Governance principles should include:
- All project meetings take place only when at least one representative of each party can attend.
- Minutes of all meetings are taken, approved by all participants, and kept on record for an agreed period after completion of the project.
- All participants receive a briefing on competition-law rules and sign a competition-compliance and non-disclosure statement.
If the project requires specific discussion of highly sensitive topics (e.g., costs, patient information, potential tendering opportunities, or R&D activities), parties should consider having a specialist competition lawyer attend those meetings..
Appendix 5: Medicines Optimisation Innovation Centre
The Medicines Optimisation Innovation Centre (MOIC) is a regional centre in Northern Ireland dedicated to driving innovation in medicines use.
MOIC combines pharmaceutical and research and development skills with technology and business acumen to achieve smarter medicines and better outcomes for patients.
With a strong patient focus, MOIC is uniquely placed to deliver better patient outcomes by developing and sharing best practice in medicines use through research, innovation, quality improvement and knowledge transfer. Optimising medicines is vital for an ageing population and the increasing complexity of medicine regimens.
MOIC works closely with partners across a range of sectors including healthcare, academia and commercial, and welcomes collaboration with industry.

Antrim Area Hospital Site
Bush Road
Antrim
BT41 2RL
-
ThemeDevolved Nations - Northern Ireland
-
KeywordsNorthern IrelandDevolved Nations - Northern IrelandNICON
-
PublisherNICON, ABPI



