Foreword
Foreword
Better health and a stronger economy go hand in hand – Scotland can have both.
Every advance in medicines – from vaccines to treatments that extend life – depends on strong partnerships between the public sector (government and the NHS), academia and industry. This ‘triple helix’ drives Scotland’s life sciences growth, contributing more than £1.7 billion to the economy and supporting 15,000 high-value jobs.1
Scotland has many of the building blocks needed to be a global leader in the research, development and use of cutting-edge medicines and diagnostics – but only if the right policy choices are made now.
Uniform and timely access to medicines can help reduce health inequalities, while innovations such as medicines for obesity create new opportunities to address the root causes of ill health.
The next Parliament offers a critical window to reset industrial and NHS innovation policy. Global competition for investment has never been higher. At the same time, health systems have never needed innovation more.
The opportunity is real – but unlocking it depends on bold choices that put research, innovation and patient access at the heart of Scotland’s health and industrial strategy.

Our ambition for Scotland
Scotland has the foundations to lead globally in life sciences. If the right choices are made, the next Parliament could deliver:
- Life sciences as a central pillar of economic growth – attracting investment and creating high-value jobs in research and development, clinical trials, advanced manufacturing and NHS partnerships.
- Better health outcomes and fewer people out of work due to ill health – through faster access to breakthrough medicines and vaccines, driving down treatable mortality.
- A world-leading digital health system – where data and genomic insights guide prevention, treatment and innovation.
- Rapid, consistent patient access to effective medicines – is what patients can expect in a healthcare system that is ambitious, agile and internationally competitive.
To turn this ambition into reality, we ask the next Parliament to:
- Guarantee timely patient access to new medicines – so that NHS clinicians and patients benefit consistently from cutting-edge treatments and vaccines.
- Support Scotland to lead in research and innovation – creating a world-class environment for globally mobile clinical trials, early-stage research and advanced manufacturing.
- Drive health and care delivery in Scotland with data and digital – ensuring responsible, interoperable and trusted use of health data for care and research.
Access to cutting-edge treatments and vaccines
The challenge
Uptake of new medicines in Scotland remains slow and uneven, particularly precision medicines including those requiring a genetic test. Patients face postcode lotteries and delays compared with other advanced health systems. Slow and variable access to new treatments risks widening health inequalities and declining health outcomes. This places greater strain on the health system, and on Scotland’s public finances due to economic inactivity driven by ill health. Scotland suffers from slower and narrower access to cutting-edge medicines than other developed nations.2
Breakdown of total availability to patients (%, 2020–2023):
The break down of total availability is the composition of medicines available to patients in European countries as of January 2025. This is based on the availability of a medicine relative to its licence.
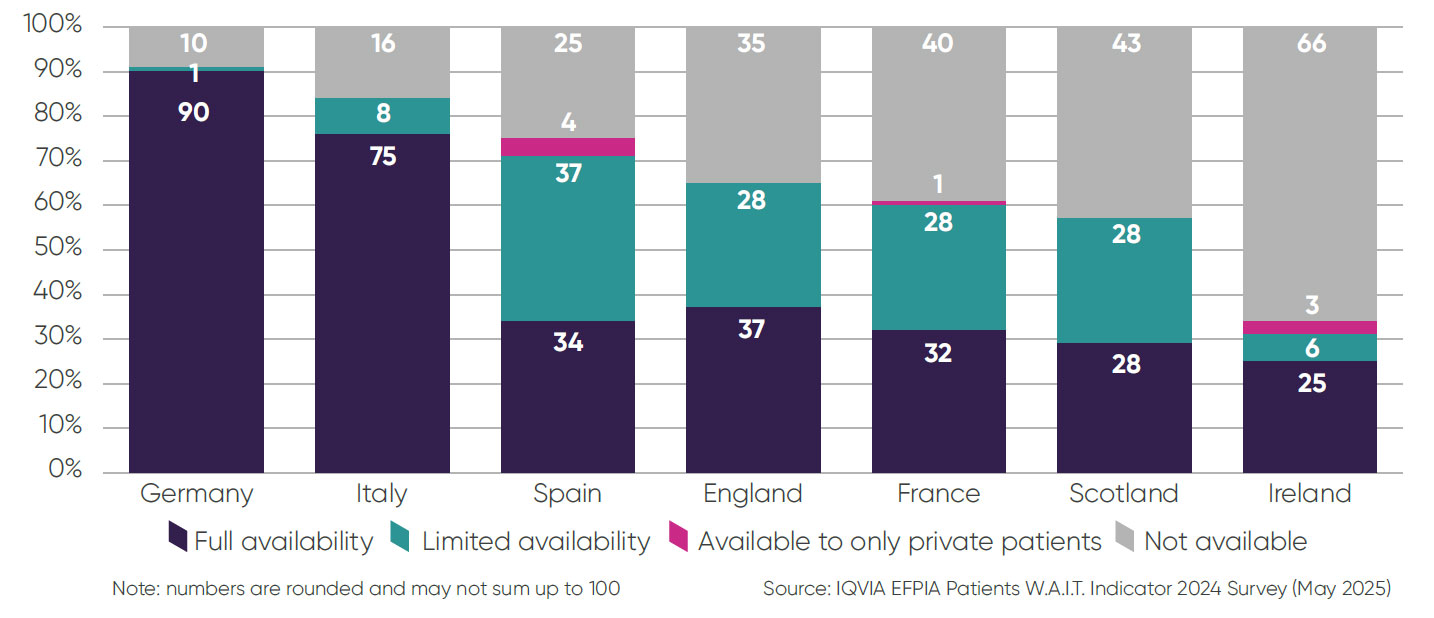
- ▶ Only 28 per cent of medicines available to European patients are fully available in line with their licence in Scotland – lower than England at 75 per cent. Germany, by comparison, has full availability rates of 100 per cent3.
- ▶ It takes an average of 374 days for a medicine to become available to prescribers in Scotland after it receives a licence4.
- ▶ Genomic medicines, which require specialist testing, face even longer delays.
- ▶ The UK is seen internationally as placing less value on innovation, investing a far lower percentage of overall healthcare spend on medicines than other developed nations5.
Driving the adoption of innovation
The Centre for Sustainable Delivery, under the Innovation Design Authority, is driving access to innovation through the Accelerated National Innovation Adoption (ANIA) assessment process and the Modernising Patient Pathways Programme. But too many conflicting local guidelines and processes create uncertainty, waste staff time and slow the spread of proven improvements that patients can benefit from. Evidence-based guidance developed elsewhere – in Scotland, the UK, or internationally – is often not adopted locally.
Our vision
Every patient should be able to access Scottish Medicines Consortium (SMC)-accepted treatments quickly, consistently and fairly. Spending on medicines must be recognised as an investment in health as well as NHS efficiency and financial sustainability. By investing in cutting-edge treatments and vaccines, we can drive disease prevention, improve treatable mortality and generate economic growth for Scotland by reducing economic inactivity.
How we get there
- ▶ Reward rapid adoption – provide financial incentives for health boards that deploy clinically effective and cost-effective innovations in line with timescales set out by the Scottish Government.
- ▶ Support a world-class SMC – ensure the SMC evolves at the forefront of Health Technology Assessment (HTA), adapting its methods to keep pace with innovation, and that it is supported by sustainable resourcing.
- ▶ Shine a light on performance – require health boards to report adoption of SMC-accepted medicines, with a comply-or-explain approach as adopted in Wales.
- ▶ Bolster the New Medicines Fund – ensure that funds paid back by the pharmaceutical industry via the Voluntary Agreement for Branded Medicines Pricing, Access and Growth (VPAG) investment fund are channelled to health boards to promote adoption and accelerated access to innovative medicines.
- ▶ Scale up the ANIA pathway – provide ring-fenced, recurring funding for innovation roll-out across Scotland.
- ▶ Remove unnecessary barriers – cut unnecessary delays, duplicative processes and reduce regional variation in access to treatment.
- ▶ Plan for the future – pilot new approaches, such as indication-based pricing, and guarantee availability of companion diagnostics for genomic medicines.
Leading in research and innovation
The UK is falling behind
- Between 2017/18 and 2022/23, commercial trial participants dropped by nearly 14,000.
- The UK has slipped from 4th to 8th globally in phase III trial delivery.8
Without urgent action, Scotland risks losing clinical trials – and with them the investment, jobs and early-patient-access opportunities they bring – to faster and more reliable competitor countries.
Industry clinical trials deliver significant value to the economy, NHS, and wider science base of Scotland and the UK
In 2022, industry clinical trials contributed:
Industry clinical trials contributed £7.4 billion of gross value added (GVA) to the UK economy and supported a total of 65,000 jobs.
Generated £1.2 billion of revenue for the NHS and supported 13,000 NHS jobs.
£0.9 billion of GVA was generated by the contribution of industry clinical trials to improved patient outcomes in research-active hospitals compared to research-inactive hospitals.
These health improvements are estimated to prevent 3 million sick days.
Over 5 years:
Industry contributed to almost 5,000 research papers between 2019 and 2023.
11% of these papers were highly cited, compared to an EU benchmark of 5.7%.
These papers supported 283 patent applications.9
The value of industry clinical trials to Scotland and the UK
Our vision
Every patient should have the opportunity to participate in research, driving better outcomes, stronger NHS capacity and economic growth.
How we get there:
- Scale triple-helix collaboration – build on the industry-funded CATALYST Programme by reinvesting NHS revenues from industry trials to create a long-term financially sustainable and world-class trials environment in Scotland.
- Increase transparency – publish site- and board-level performance data on commercial trial delivery, alongside reporting of associated income where appropriate, to highlight strengths and drive improvement.
- Adopt ‘Once for Scotland’ approvals and deliver the fastest set-up and most reliable patient recruitment in the UK – replicate COVID-era speed with trial setup and NHS data access in days; matching or exceeding England’s plan for a 150-day clinical trial set-up time.
- Strengthen NHS–industry partnerships – encourage NHS/industry collaborations by embedding new partnership guidance [A Common Understanding 2025] across all health boards.
- Improve horizon scanning for future innovations – evolve the horizon scanning advisory board into a single front door for innovation, enabling early dialogue between industry and NHS Scotland on all new medicines, including diagnostics, service design and patient pathways, so all appropriate patients can benefit without delay.
- Showcase Scotland’s strengths – map unique capabilities across the ecosystem, from pre-clinical research to advanced manufacturing, and market this to prospective investors.10

Powering Scotland's health future through effective use of health data
The challenge
Health data can transform diagnosis, treatment and monitoring – and Scotland’s cradle-to-grave datasets offer a chance to attract investment, advance research and improve patient outcomes. This should go hand-in-hand with delivering the Genomic Medicine Strategy11 and strengthening data infrastructure, shaped by industry input on end-user needs, genomic testing and proportionate data access.
However, data is often fragmented, hard to access, and underused – a missed opportunity for patient care and for Scotland’s leadership in life sciences. The removal of the Quality Outcomes Framework and lack of a national GP data platform have left gaps in tracking chronic conditions. Scotland’s Health and Social Care Data Strategy rightly recognises the value of responsible data use, but avoidable barriers still hinder safe, ethical access for research and innovation, risking the confidence of the public, the research community and investors.
Our vision
Patient outcomes are recorded and curated as a national priority, with appropriately governed datasets made accessible to approved academic and industry researchers.
How we get there
- Drive a national data strategy: the Health Secretary should lead a ‘Once for Scotland’ approach to health data – enabling safe, efficient use for research and innovation – with delivery led by the Chief Scientist for Health.
- Rebuild core infrastructure: restore condition reporting in primary care, invest in disease registries and create a single metadata directory of NHS datasets to enable meaningful analysis and insight.
- Enable access and build trust:
-
- Streamline and simplify data access by the pharmaceutical industry to Scottish health data (in ways acceptable to the public and healthcare professionals).
- Ensure research access to comprehensive Scottish GP data, linked to the national datasets already available for research.
- Pioneer approaches for data access, linkage and governance that can inform the new UK Health Data Research Service and ensure Scotland maximises its benefit, including a single point of entry for global companies to access Scottish health data across the country.
Our asks of Scotland's leaders
1. Faster access to innovation
Require every health board to report on access performance for SMC-approved medicines, with a comply-or-explain standard and international benchmarking.
2. A world-class research environment
Scotland is benefiting from the VPAG investment fund to build its clinical trial infrastructure. Recognise that clinical trials for new medicines are placed where current best ‘standard-of-care’ medicines are routinely available to patients. Nurture triple-helix partnership working between industry, academia and government, and encourage transparent reporting of trial performance and income.
3. Consistent adoption of proven innovations
Apply ‘Once for Scotland’ pathways, protocols and guidelines, supported by recurring funding for the ANIA.
4. A data-driven NHS
Deliver a national health data strategy with restored primary-care reporting, strengthened disease registries and a single metadata directory.
Endnotes
- Fraser of Allander Institute, ‘The economic contribution of the
Pharmaceuticals Sector in Scotland’, February 2023. - EFPIA, ‘Patients W.A.I.T. Indicator 2024 Survey’, May 2025.
- Ibid page 16.
- Ibid page 17.
- ABPI, UK Competitiveness indicators, September 2025
- Scottish Government guidance (2010,2011 and 2012).
- Indication-based pricing of medicines is a reimbursement approach
where the price or level of payment for a medicine can be varied to reflect the level of unmet need, and the value to patients and the NHS of that medicine used in the specific clinical circumstances (indication) for which it is licensed. - Ibid page 11.
- ABPI, Value of industry clinical trials to the UK - extended report,
Dec 2024, p3. - ABPI Sector Insights Map.
- Scottish Government, ‘Genomic medicine strategy 2024 to 2029’, April 2024.
-
ThemeDevolved Nations - Scotland
-
KeywordsDevolved Nations - Scotland
-
PublisherABPI

