Use lessons from COVID-19 to revitalise post-pandemic clinical research environment, says ABPI
The negative impact of the pandemic on the nation’s clinical research environment can be transformed across every part of the UK if we adopt the innovative approaches taken to tackling COVID-19.
The key to unlocking the benefits of clinical research for all patients is properly resourcing and embedding a culture of research into an NHS which has been overwhelmed by the pandemic, says a new report by the ABPI.
The pandemic has demonstrated the UK’s massive potential as a global leader in clinical research with 68 commercial COVID-19 trials initiated in 2020– the most in Europe and third only to the United States and Brazil.
However, the focus on COVID-19 has negatively impacted on research across many other diseases – including cancer, heart disease and diabetes - with clinical trials paused during the early stages of the pandemic and continued disruption since.
The UK’s research efforts are also recovering at a slower pace than some other similar countries in Europe, including Spain and Italy, who were also hit hard in the pandemic.
The report also shows that the UK has fallen down the global rankings in all phases of clinical trials in 2020.
We are proud of the achievements in COVID-19 research, but we must now learn from them if we are to rebuild and transform the UK’s whole clinical research offering. Richard Torbett, ABPI Chief Executive
We fully support ABPI’s recommendation to increase and diversify patient recruitment to clinical trials and look forward to continuing work with them to help build a future where everyone across the UK can take part in and benefit from the results of the best possible clinical research. Hilary Reynolds, CEO of the Association of Medical Research Charities
Putting research at the heart of the NHS’s recovery will help deliver better outcomes for patients, improve job satisfaction within the workforce and benefit the UK economy. It must be a key priority for policy makers as we emerge from the pandemic. Professor Ramesh Arasaradnam, Academic Vice-President of the Royal College of Physicians
While these times have been challenging we have learnt much about the ‘art of the possible’ in research delivery and are determined to build from that to develop a brighter future for the UK clinical research ecosystem. William van't Hoff, Chief Executive of the National Institute for Health Research Clinical Research Network
The figures are from the ABPI’s third annual report: Clinical research in the UK: an opportunity for growth, which makes recommendations to rebuild and transform the UK’s clinical research offering.
Adopting innovative research design and delivery approaches, as seen during the COVID-19 pandemic, fixing bottlenecks in the system, and embedding a culture of research across the health service, will not only benefit the NHS and its patients, but significantly contribute to the Government’s ambition to level up the country and address health inequalities, say authors.
Among the headline figures:
COVID-19 research
- The UK led Europe with 68 commercial COVID-19 trials initiated in 2020, third only behind Brazil and the USA.
- In 2020/21 1,390,483 patients took part in clinical research in England (double 2019/20) with 905,790 in COVID-19 related studies.
- 100 per cent of hospital trusts and 50 per cent of GP practises were involved in recruitment of COVID-19 patients for studies in England
- Devolved nations recruitment was also significant with 66,000 patients In Scotland: 26,000 in Northern Ireland and 5,800 in Wales
Restart of non-COVID-19 research
- More than 40% of NHS trusts had non-COVID-19 research studies paused during the first wave of the pandemic, preventing patients receiving potentially life-saving treatments.
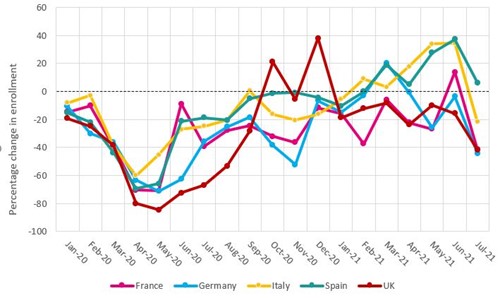
Data from a sample of commercial studies shows that the number of patients enrolled in studies in the UK has been disrupted during the pandemic:
- In May 2020, overall clinical trial enrolment was at its lowest, down 84per cent compared to May 2019.
- Oncology, which comprises the majority of the UK’s research portfolio, was the most affected, with enrolment in May 2020 down 88per cent versus May 2019.
Other countries also saw a drop in research activity during the pandemic, but not to the same extent as the UK. For example:
- France, Germany and Spain saw overall enrolment down between 66-70per cent in May 2020, and in Italy, where the first wave of the pandemic was more severe, enrolment was most impacted in April 2020, down 60per cent versus April 2019.
- The UK continues to struggle to recover enrolment in commercial studies.
- Italy and Spain have recovered their non-COVID-19 research activity the fastest, with enrolment in June 2021 37% and 34% higher than in June 2019, respectively.
- The UK on the other hand has been unable to recover enrolment to pre-COVID-19 levels, with enrolment in June 2021 15% lower than in June 2019.
- Enrolment in July 2021 across all countries has been lower than in the preceding months, which may be due to disruption caused by the COVID-19 Delta variant.
Global clinical trial rankings (full table below)
- The UK is strong in early phase clinical research and maintains its place as number one in Europe for Phase 1 clinical trial activity, with 89 trials initiated, although this is down from 160 in 2014 - a decreasing trend also seen in other European countries.
- Globally, the US takes top spot with 512 trials, with China and Australia in second and third place.
- For Phase 2 the UK ranks 5th globally - third in Europe behind Germany and Spain - with 201 initiated; USA ranks top with 953.
- However, for later stage Phase 3 trials - which see larger numbers of patients treated with potentially life-saving treatments – the UK drops behind a number of European countries – with Germany, Spain, Italy and France all placing higher – and falls to 7th
- The figures show a drop in the rankings for the UK across all Phases since 2018.
Impact on the NHS
Commercial clinical research contributes significant economic benefits, generating an estimated income of £355 million for the NHS in England in 2018/2019 and a total estimated cost saving to the NHS of £28.6 million in the same year.
In contrast, the loss of commercial research across NHS Trusts during the pandemic is estimated to have generated a loss to the NHS of up to £447 million in total in 2020/2021.
Recommendations
The report makes five recommendations which are needed if the UK is to revitalise its research base for UK patients and compete globally for life-science investment.
- Embed clinical research in healthcare
- The new Health and Care Bill must mandate that Integrated Care Systems ensure that NHS organisations, for which they are responsible, conduct and resource clinical research.
This will benefit all NHS Trusts and patients across the UK, by encouraging fair access for all patients to medical research, improve enrolment on trials and boost the Government’s levelling up agenda and commitment to tackling health inequalities.
- Reform and streamline approvals and set up of clinical trials
- Use the Medicines and Medical Devices Act 2021 to mandate rapid timelines for regulatory approvals and reform clinical trial regulation. Ensure costing and contracting processes being made quicker, more transparent and less variable.
- Ensure that the MHRA and HRA are properly resourced.
The upcoming spending review is the opportunity to ensure that the MHRA and HRA are properly resourced to deliver against the UK’s ambition for clinical trials, as set out in the Life Sciences Vision.
- Increase and diversify patient recruitment to clinical trials
- Clinical research activity should be scaled-up across the healthcare system in primary, secondary and tertiary care settings
- System partners should work together to pilot community-based projects to support local healthcare systems in building relationships with underserved communities and understanding unmet need relative to local and national demographics.
- Despite overwhelming evidence that COVID-19 disproportionately affects people from ethnic minority backgrounds, only 9 per cent of participants involved in COVID-19 studies across the UK were from these backgrounds. With a diverse population of 60 million the UK has the potential for improvement.
- Adopt innovative clinical trial design and delivery approaches Develop and put into practice standardised approaches and guidance for innovative design and delivery trials. Deliver training on innovative design and delivery approaches for ethics committees, regulators, researchers involved in trial design and delivery teams.
- Ensure that digital tools are available to support remote and virtual trial delivery, in particular availability of electronic health records.
- The search for COVID vaccines and treatments saw regulatory flexibilities introduced, digital and remote approaches adopted, and innovative design and delivery models implemented which enabled COVID-19 and non-COVID-19 research to be conducted during the pandemic.
- Improve how the UK reports on clinical research performance
- UK Government to work with system partners on the development of a UK-wide clinical research dashboard, agreeing the metrics needed to articulate performance across the UK healthcare system and evidence the impact on patients, the NHS and the economy.
ABPI Chief Executive Richard Torbett said:
“The report paints a mixed picture on clinical research here in the UK. We are proud of the achievements in COVID-19 research, but we must now learn from them if we are to rebuild and transform the UK’s whole clinical research offering.
“The value of clinical research has never been more evident – it is the key to global recovery, improving public health and protecting us from future pandemics, but it can only be achieved if we support the NHS to embed a culture of research across the UK.”
“The Government’s UK-wide vision for clinical research delivery aims to rebuild post-pandemic and create an ecosystem which is globally competitive, brings value to the NHS and benefits all patients. What matters now is that these commitments are translated into real action.”
Hilary Reynolds, CEO of the Association of Medical Research Charities (AMRC) said:
“Although we’re encouraged by the attractiveness of the UK to conduct clinical research highlighted in this report, it is concerning to see that we’re lagging behind our international competitors in the recovery of that research post-COVID.
“It is clear that substantial and sustained investment and collaboration across the research and healthcare systems is required, with an active and resourced commitment from Government to fulfil their aim for the UK to be a science superpower.
“We fully support ABPI’s recommendation to increase and diversify patient recruitment to clinical trials and look forward to continuing work with them to help build a future where everyone across the UK can take part in and benefit from the results of the best possible clinical research."
“Last year really demonstrated the high value and impact of the UK clinical research system. By collaborating across the system and innovating, the UK played a leading role in delivering commercial COVID-19 research, with outcomes benefitting the world.
Dr William van’t Hoff, Chief Executive of the National Institute for Health Research Clinical Research Network said:
“Inevitably, pivoting towards COVID-19 reduced our ability to deliver non-COVID research but as the COVID vaccine programme has progressed, we have redirected our focus to fully restoring and building back our entire portfolio of research.
“The experience of COVID has shown us what we can achieve when research is embedded in our health and care system and we need to build on this going forward and achieve the ambitions set out in the Clinical Research Delivery Vision - using data to optimise study placement, building research capacity and ensuring that research participants represent the UK’s diverse population.
“While these times have been challenging we have learnt much about the ‘art of the possible’ in research delivery and are determined to build from that to develop a brighter future for the UK clinical research ecosystem.”
Professor Ramesh Arasaradnam, Academic Vice-President of the Royal College of Physicians said:
“This report highlights that there are major opportunities for clinical research in the wake of the pandemic, but also some significant challenges. Clinical trials have played a crucial role in the development of new vaccines and treatments for COVID-19, demonstrating the importance of research for all to see. At the same time, the disruption that the pandemic has caused to other areas of clinical research is very concerning.
“It is imperative that we return levels of research activity to at least what they were before the pandemic. Participating in non-COVID-19 trials is potentially transformative for patients’ health and wellbeing.
“Equally, we know that clinicians themselves really value the chance to contribute to clinical research. 57% of our members would like to be more involved in research, and we must tap into this considerable enthusiasm.
“Putting research at the heart of the NHS’s recovery will help deliver better outcomes for patients, improve job satisfaction within the workforce and benefit the UK economy. It must be a key priority for policy makers as we emerge from the pandemic.”
Russell Abberley, ABPI Board Member and General Manager UK/Ireland at Amgen said:
“Global pharmaceutical companies are looking for the optimal environment to conduct their clinical trials; places where they can cultivate strategic partnerships and innovate in clinical development in a timely manner. The UK needs to demonstrate it can deliver that.
“Putting the recommendations of this report into action will help make the UK a leading destination for researching and developing new medicines, with compelling treatment benefits for patients and economic advantages for the NHS.”
Key data
Global rankings - From 2021 report Clinical research in the UK: An opportunity for growth
Number of clinical trials initiated in 2020 by phase and country
|
# |
Country |
Phase I |
Country |
Phase II |
Country |
Phase III |
|
1 |
USA |
512 |
USA |
953 |
USA |
537 |
|
2 |
China |
343 |
China |
361 |
Spain |
273 |
|
3 |
Australia |
117 |
Germany |
213 |
Germany |
264 |
|
4 |
UK |
83 |
Spain |
203 |
China |
241 |
|
5 |
Japan |
83 |
UK |
201 |
Italy |
229 |
|
6 |
Spain |
73 |
France |
164 |
France |
226 |
|
7 |
Germany |
66 |
Japan |
164 |
UK |
224 |
|
8 |
France |
49 |
Canada |
151 |
Canada |
220 |
|
9 |
Canada |
47 |
Australia |
146 |
Japan |
218 |
|
10 |
Belgium |
35 |
Italy |
132 |
Poland |
194 |
|
11 |
Italy |
20 |
Poland |
113 |
Australia |
181 |
|
12 |
Poland |
10 |
Belgium |
97 |
Belgium |
158 |
|
13 |
Switzerland |
8 |
Hungary |
58 |
Hungary |
152 |
|
14 |
Brazil |
7 |
Brazil |
49 |
Brazil |
151 |
|
15 |
South Africa |
3 |
Switzerland |
35 |
Switzerland |
67 |
|
16 |
Hungary |
2 |
South Africa |
25 |
South Africa |
48 |
Global rankings – From 2020 report Clinical trials: How the UK can transform the clinical research environment
Number of clinical trials initiated in 2018 by phase and country
|
# |
Country |
Phase I |
Country |
Phase II |
Country |
Phase III |
|
1 |
USA |
526 |
USA |
1021 |
USA |
626 |
|
2 |
China |
234 |
UK |
268 |
Germany |
315 |
|
3 |
UK |
95 |
Germany |
249 |
Spain |
308 |
|
4 |
Germany |
68 |
Spain |
221 |
UK |
292 |
|
5 |
Australia |
66 |
France |
200 |
France |
290 |
|
6 |
Japan |
66 |
Japan |
198 |
Canada |
289 |
|
7 |
Spain |
49 |
China |
190 |
Italy |
264 |
|
8 |
Canada |
43 |
Canada |
187 |
Poland |
250 |
|
9 |
Belgium |
39 |
Italy |
157 |
Japan |
242 |
|
10 |
France |
37 |
Australia |
152 |
China |
219 |
|
11 |
Italy |
20 |
Poland |
133 |
Australia |
211 |
|
12 |
Poland |
10 |
Belgium |
125 |
Belgium |
208 |
|
13 |
South Africa |
6 |
Hungary |
74 |
Hungary |
177 |
|
14 |
Switzerland |
6 |
Switzerland |
56 |
Brazil |
123 |
|
15 |
Hungary |
2 |
Brazil |
37 |
Switzerland |
101 |
|
16 |
Brazil |
No data |
South Africa |
20 |
South Africa |
77 |
Percentage change in total enrolment for commercial studies per month relative to 2019 baseline, by country (January 2020-July 2021)
Access the interactive graphs and data on the ABPI website.
Last modified: 20 September 2023
Last reviewed: 20 September 2023
-
Clinical research in the UK: an opportunity for growth
Download
